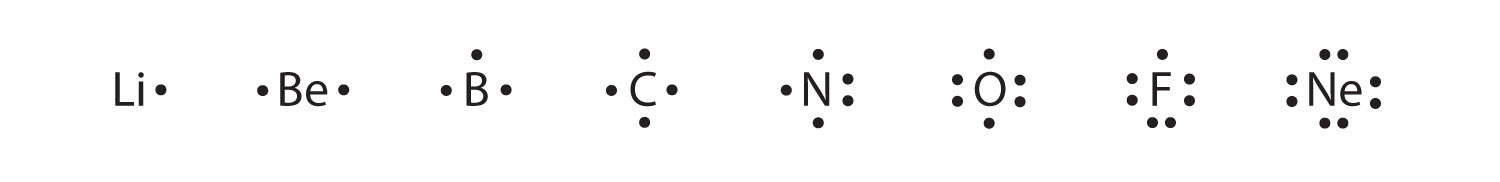Profil Be3N2 Lewis Structure Terbaru. It is an ionic compound so it would not have a lewis dot structure. Atoms and the periodic table unit project for this unit project, you will be conducting more research.

We have discussed the various types of bonds that form between atoms and/or ions.
When you draw lewis structures of ions, you have to draw the cation and the anion separately, enclose them in square brackets, and indicate their respective coefficients and charges. Atoms and the periodic table unit project for this unit project, you will be conducting more research. N₂o has three resonance forms whose structures are given below. Because this lewis structure has only 6 electrons around the central nitrogen, a lone pair of electrons on a terminal atom must be used to form a bonding pair.